6.1. Fundamentals#
import matplotlib
import matplotlib.pyplot as plt
import numpy as np
from IPython.display import display
from PIL import Image
import os
# check for set environment variable JB_NOSHOW
show = True
if 'JB_NOSHOW' in os.environ:
show = False
As already outlined, there exist three heat transfer modes. All of them are important for the modelling of fires.
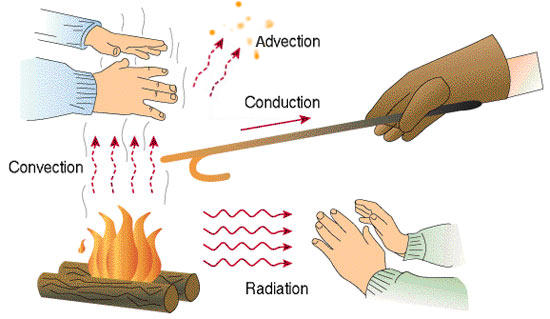
Fig. 6.1 Schematic illustration of the various heat transfer modes. Source: Wikimedia Commons.#
conduction: gas phase near the flame, heating of solids (critical structures or solid fuel sources)
convection: transport of hot smoke in buildings, heat flux from hot gas to building structures
radiation: heating of walls and fuel sources due to hot gases and soot
Heat Conduction#
The heat conduction in solids is different for (electric) insulators and (electric) conductors. While there are no free electrons in an insulator the conduction is mainly due to phonons, i.e. atom oscillations in the ordered lattice.
In addition to this transport mechanism, the free electrons in conductors allow to significantly increase the heat conducting capacity. Therefore, (electric) conductors are better heat conductors than (electric) insulators.
Fig. 6.2 Illustrative representation of heat conduction in a solid. Particle ensembles with high temperature (red) have higher oscillation amplitutes as those at medium (yellow) or low (blue) temperature.#
In gases and fluids, particles are not in fixed positions. They collide with each other and thus transfer energy and momentum. If the mean free length is small (w.r.t. the system size) then the collisions tend to establish an equilibrium.
A general global picture is as follows:
Hot parts of a gas contain particles with high (thermal) kinetic energy,
cold parts have slower particles,
due to collisions, both distributions tend to equalise and therefore there is an heat flow from the warm to the cold portions of the gas.
Fig. 6.3 Illustrative representation of heat conduction in a fluid. Particle from warm regions have more (thermal) kinetic energy and transfer it to particles with lower one.#
In general, all heat conduction processes may be modelled with the (differential) Fourier’s law
where \(\mf \vec{q}\) is the conduction vector, \(\mf \mathbf{k}\) is the conduction matrix and \(\mf T\) the temperature field. In general the conduction is neither isotropic nor homogenous, therefore a scalar heat conduction coefficient is not sufficient. Additionally, it depends on the temperature.
The integral form describes the heat transfer rate through an surface \(\mf S\) (given a constant scalar \(\mf k\))
The mathematical description of heat conduction is given by the heat equation for the temperature \(\mf T=T(\vec{x}, t)\). In solids, there is no convection, thus this is the only equation needed to determine the temperature.
In absence of work done, the change of the heat content \(\Delta Q\) is directly proportional to the change in temperature \(\Delta T\):
Therefore conduction coefficient in the above parabolic PDE is given by
Thermally thin materials have a short balancing time (e.g. due to high conductivity) and react directly to changing boundary conditions. The temperature profiles are in general linear.
Thermally thick materials propagate the changes in boundary conditions on a longer time scale as the boundary values may change. Therefore the temperature profile is in general of non-linear nature.
Fig. 6.4 Schematics of the temperature profile in a one dimensional setup. The heat flux is based on the temperature difference between the heated side (\(\mf T_0\)) and the backward side (\(\mf T_B\)). Thermally thin and thick materials have a different temporal scales.#
Convective Heat Transfer#
Fluid Phase#
The convective heat transfer is not a principle physical phenomenon. The transfer is due to medium movement which is driven by density fluctuations in a gravitational field.
There exist basically two regimes for convective heat transfer:
natural: the density changes are driving the mediums motion, and
forced: an external source moves the medium.
Fig. 6.5 General representation of the convective heat transfer. Source: Wikimedia Commons.#
Fluid-Solid Interface#
The heat flux \(\mf q\) through a surface \(\mf A\) is given by the temperature difference of the gas \(\mf T_g\) and the solid wall \(\mf T_w\), by Newton’s cooling law
with the heat transfer coefficient \(\mf h\). Although the law is simple, the determination of the heat transfer coefficient is complex.
The Nusselt \(\mf Nu\) number is the ratio of convective to conductive heat transfer
with a given characteristic length scale \(\mf L\) and the heat conductivity \(\mf k\). In general, the Nusselt number is a function of other dimensionless numbers (Reynolds \(\mf Re\) and Raylight \(\mf Ra\)):
forced convection: \(\mf Nu = f(Re, Pr)\)
natural convection: \(\mf Nu = f(Ra, Pr)\)
The individual forms of the function strongly depend on the geometry. There exist many collections in the literature, where generalised cases with explicit formulations are stated. For example, see chapter 3 (Convection Heat Transfer) in [SFPE16].
Thermal Radiation#
Electromagnetic Waves#
The physical principles of radiative heat transfer is the fact, that all matter with finite temperature emit electromagnetic waves, which travel with the speed of light (\(\mf c\approx 3\cdot 10^8 m/s\)). An overview of the electromagnetic spectrum is given in Fig. 6.6.
Fig. 6.6 The electromagnetic spectrum. Source: Wikimedia Commons.#
The emitted energy spectrum, \(\mf I\) as a funktion of the wave frequency \(\mf \nu\), of the so called black body radiation is given by Planck’s law
for a finite temperature \(\mf T\). Here \(\mf h=6.63\cdot10^{-34}Js\) denotes Planck’s constant. This thermal spectrum is illustrated for a few temperatures in Fig. 6.7.
def mono_em_power(temperature, wavelength=None):
"""
Monochromatic emissive power
Enclosure Fire Dynamics, B. Karlsson and J. G. Quintiere,
2000 by CRC Press LLC, Formula 7.3
:param temperature: thermodynamic temperature, in K
:param wavelength: wave length, in µm
:return: emissive power, in W/m2-µm
"""
C1 = (3.743 * 10 ** 8) # W*µm**4/m**2
C2 = (1.4387 * 10 ** 4) # µm * K
wavelength = wavelength
emissive_power = (C1 * wavelength ** -5) / (
np.e ** (C2 / (wavelength * temperature)) - 1)
return emissive_power
def wavelength_to_rgb(wavelength, gamma=0.8):
'''
taken from https://stackoverflow.com/questions/44959955/matplotlib-color-under-curve-based-on-spectral-color
taken from http://www.noah.org/wiki/Wavelength_to_RGB_in_Python
This converts a given wavelength of light to an
approximate RGB color value. The wavelength must be given
in nanometers in the range from 380 nm through 750 nm
(789 THz through 400 THz).
Based on code by Dan Bruton
http://www.physics.sfasu.edu/astro/color/spectra.html
Additionally alpha value set to 0.5 outside range
'''
scale = 1#/1000
wavelength = float(wavelength)
if wavelength >= 380*scale and wavelength <= 750*scale:
A = 1.
else:
A=0.5
if wavelength < 380*scale:
wavelength = 380.*scale
if wavelength >750*scale:
wavelength = 750.*scale
if wavelength >= 380*scale and wavelength <= 440*scale:
attenuation = 0.3 + 0.7 * (wavelength - 380*scale) / (440*scale - 380*scale)
R = ((-(wavelength - 440*scale) / (440*scale - 380*scale)) * attenuation) ** gamma
G = 0.0
B = (1.0 * attenuation) ** gamma
elif wavelength >= 440*scale and wavelength <= 490*scale:
R = 0.0
G = ((wavelength - 440*scale) / (490*scale - 440*scale)) ** gamma
B = 1.0
elif wavelength >= 490*scale and wavelength <= 510*scale:
R = 0.0
G = 1.0
B = (-(wavelength - 510*scale) / (510*scale - 490*scale)) ** gamma
elif wavelength >= 510*scale and wavelength <= 580*scale:
R = ((wavelength - 510*scale) / (580*scale - 510*scale)) ** gamma
G = 1.0
B = 0.0
elif wavelength >= 580*scale and wavelength <= 645*scale:
R = 1.0
G = (-(wavelength - 645*scale) / (645*scale - 580*scale)) ** gamma
B = 0.0
elif wavelength >= 645*scale and wavelength <= 750*scale:
attenuation = 0.3 + 0.7 * (750*scale - wavelength) / (750*scale - 645*scale)
R = (1.0 * attenuation) ** gamma
G = 0.0
B = 0.0
else:
R = 0.0
G = 0.0
B = 0.0
return (R,G,B,A)
# Wave length band in µm.
wave_length_band = np.linspace(0.1,10,1000)
wavelengths = np.linspace(100, 1000, 1000)
temperatures = [500, 1000, 1500]
#300, 400,
#500, 600, #1000]
#500, 700, 800, 900, 1000,
#1100, 1200, 1300, 1400]
# Define the wave length for the visual spectrum in nm.
clim=(350,780)
# clim=(0.350,0.780)
# Scale the colors to the to the correct x-values (wave length).
norm = plt.Normalize(*clim)
wl = np.arange(clim[0],clim[1]+1,2)
# wl = np.arange(clim[0],clim[1]+0.001,0.002)
colorlist = list(zip(norm(wl),[wavelength_to_rgb(w) for w in wl]))
spectralmap = matplotlib.colors.LinearSegmentedColormap.from_list("spectrum", colorlist)
# Set up plot.
fig, axs = plt.subplots(1, 1, figsize=(8,4),
tight_layout=True)
# Create map to fill the background with the colour spectrum.
# y = np.linspace(0, 6, 100)
y = np.linspace(0, 100, 100)
X,Y = np.meshgrid(wavelengths, y)
extent=(np.min(wavelengths),
np.max(wavelengths),
np.min(y), np.max(y))
# Plot the colour spectrum.
plt.imshow(X, clim=clim, extent=extent,
cmap=spectralmap, aspect='auto',
alpha=0.3)
# Plot the emission curves based on radiator temperature.
for radiator_temp in temperatures[:]:
em_powers = list()
for wavelength in wave_length_band:
em_power = mono_em_power(temperature=radiator_temp,
wavelength=wavelength)
em_powers.append(em_power/1000)
plt.plot(wave_length_band*1000, em_powers,
label="{} K".format(radiator_temp), lw=2)
plt.xlabel('Wave Length / $\sf nm$')
plt.ylabel('Intensity / $\sf W/(m^2\cdot nm)$')
plt.xlim()
plt.grid()
plt.legend()
plt.savefig('./figs/radiative-spectrum-full.svg')
if show: plt.show();
plt.clf();
Fig. 6.7 Radiative emission spectrum.#
With higher surface temperature
the maximal intensity wavelength moves to lower values, and
the total intensity increases.
Fig. 6.8 Approximation of the color of an incandescent object. Source: Wikimedia Commons.#

Fig. 6.9 Hot metal. Source: Wikimedia Commons.#
Emitted Power#
The total energy (\(\mf E_b\)) emitted by a black body radiator is described by the Stefan-Boltzmann law
with the Stefan-Boltzmann constant \(\mf \sigma = 5.67\cdot 10^{-8}W~m^{-2}K^{-4}\). However, in practical applications most radiation comes from non-ideal black body radiators, and therefore the concept of gray bodies is introduced. The ratio of energy emitted to the emission of a fictitious \(\mf \epsilon\) is defined as
and is called emissivity. In this assumption it is independent of the emitted wavelength. Absorptivity is equal to emissivity.
There exist three ways how an incident electromagnetic wave can interact at the surface of a gas and a solid body, see Fig. 6.10:
absorption: the wave’s energy is transferred to heat
transmission: the wave passes the body
reflection: the wave is reflected either specular or diffusive
In fires, most of the reflection is a diffusive one, which means, that the reflected radiation is distributed on all outgoing angles.
Fig. 6.10 Interaction of incident radiation with a solid body.#
The energy flux \(\mf Q_{1\rightarrow 2}\) from a diffusive radiator surface \(\mf A_1\) to an other surface \(\mf A_2\) is given by
with the view factor \(\mf F_{1\rightarrow 2}\). This factor is the fraction of the totally emitted energy that may be absorped by the target, here the surface \(\mf A_2\). Fig. 6.11 illustrates the geometrical setup of the following view factor integration:
Fig. 6.11 General view factor between infinitesimal surface elements.#
There exist integrated results for many configurations, like shown in the appendix D (Configuration Factors) in [SFPE16].
Radiation Transfer#
The transport of radiation through a medium must include the following processes:
absorption,
scattering,
emission, and
scattering addition.
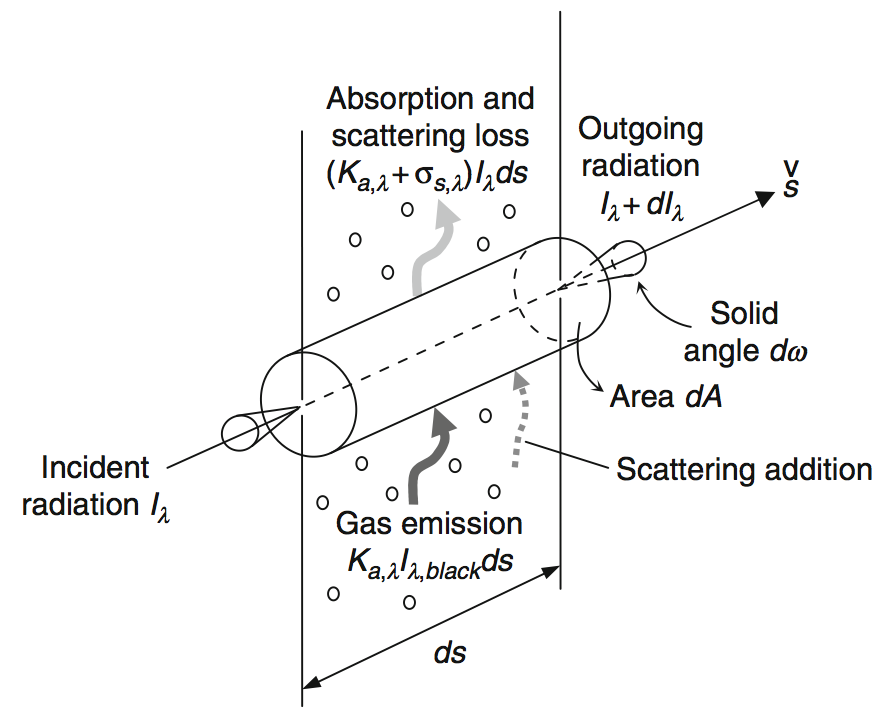
The change of monochromatic intensity \(\mf I_\lambda\) in a control volume in the direction \(\mf \vec{s}\) is given by
Due to the fact, that light travels so fast, a common approximation is to neglect the partial derivative w.r.t. time:
The change of intensity per unit path length is due to net gain of radiant energy and therefore:
This equation involves coefficients for local spectral absorption (\(\mf K_{a,\lambda}\)) and scattering (\(\mf \sigma_{a,\lambda}\)). The scattering phase function \(\mf \Phi\) allows to integrate over a full sphere to compute scattered waves into the direction \(\mf \vec{s}\). As this integral differential equation is very difficult to solve in complex geometries, a common assumption in field modelling is to neglect scattering effects. This reduces the transport equation to an ordinary differential equation:
Gases interact differently with radiation w.r.t. to the wavelength:
molecular gases (e.g. \(\mf H_2O\) or \(\mf CO_2\)) are selective absorbers and emitters, and
large particles (e.g. soot) have a broad absorption spectrum.
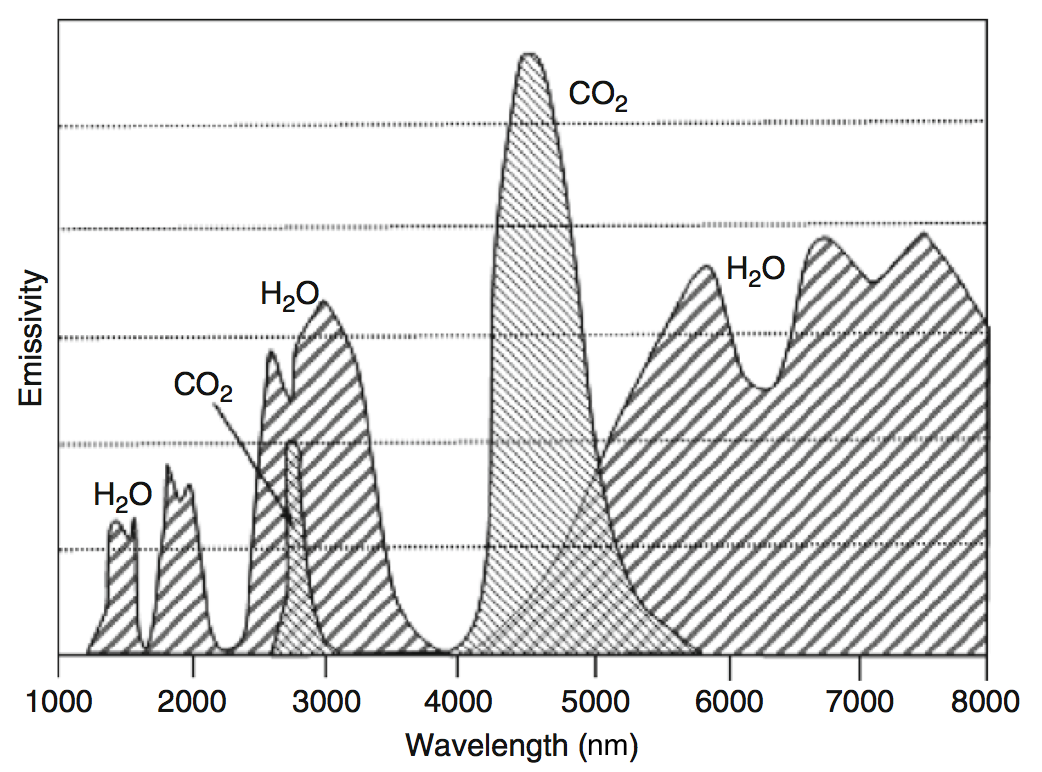
Fig. 6.13 Emissivity spectrum of \(\mf CO_2\) and \(\mf H_2O\). Source: [YY09].#
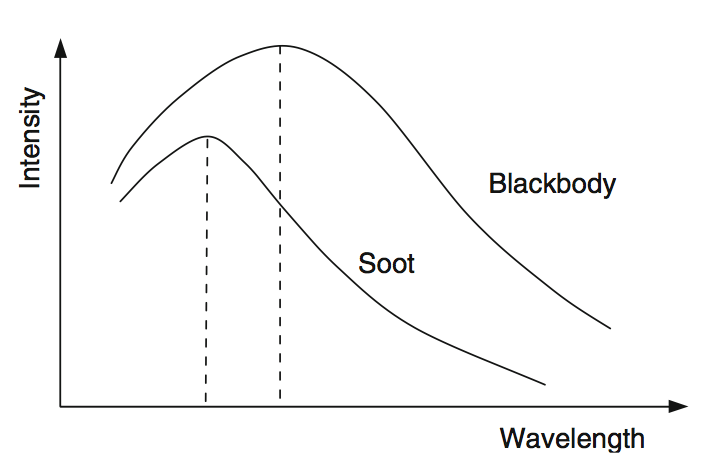
Fig. 6.14 Emissivity spectrum of soot compared to a black body radiator. Source: [YY09].#

